Microbiota as trigger of autoimmunity
Since 2012 I have established a novel research project concerning the interface between immunity and gut microbiota, which relies on my scientific knowledge regarding environmental factors, inflammation, immune-regulation and pro-inflammatory T cell responses and makes use of my numerous technical expertises in molecular biology, protein engineering (phage display), clinical immunology (T and B cell analysis, antibody analysis), bioinformatics (sequence analysis) and biostatistics. More precisely I decipher the association between gut microbiota composition and adaptive immunity with particular emphasis on the size and specificity of intestinal immunoglobulin A responses in healthy controls. I finally investigate if Multiple Sclerosis (MS) patients have aberrant gut microbiota composition and/or gut immunity. The aim is to identify commensal gut bacteria inducing specific immune responses and elucidate if such strains influence autoimmune pathology.
Host-microbiota Interactions
Throughout evolution eucaryotic organisms have been surrounded by incredible diverse consortia of foreign organisms. Eucaryotes have developed spectacular ways not only to protect themselves from pathogens, but more interestingly also ways to benefit from unique and essential features of surrounding organisms (symbionts). Whereas pathogens have been intensively studied due to their clinical impact, much less interest has been attributed to the study of symbionts. In recent years large collaborative projects have shed new light on the impact of symbionts on human health. Interestingly, bacteria acting as symbionts in one clinical setting may act as pathogens in another. Such bacteria are referred to as “pathobionts”.1
Mammals are highly dependent on their consortia of symbionts (microbiota) that serve both to optimize processing of nutrients and to protect from opportunistic agents by competition. It is also becoming increasingly clear that microbiota is critical for development of host immunity.2 Gut microbiota have exerted a strong evolutionary pressure on the gastrointestinal (GI) tract to enable it to balance the benefits and dangers of microbes. Indeed, the lower GI tract of mammals is home to an extremely dense population of gut microbiota. The GI tract employs 3 layers of control: 1) a physical barrier of specialized epithelia with tight junctions; 2) secreted mucus, anti-microbial peptides (defensins) and IgA lining the intestinal lumen and 3) innate and adaptive immune cells of the lamina propria and mesenteric lymph nodes. Therefore, despite the dense bacterial consortia inhabiting the gut, only very limited numbers of bacteria cross the epithelial barrier.
Adaptive immunity and microbes
Innate immune mechanisms controlling the mutualism between host and gut microbiota are physically localized but largely non-specific. Indeed, breach of the gut mucosal firewall leads to an immediate non-specific host response involving the secretion of defensins and the intra-luminal recruitment of innate immune cells, such as neutrophils that encapsulate commensals and limit their contact with surrounding gut epithelium.3 Whereas the active control is crucial for the fitness of the host it comes with a cost with regards to fitness of the commensal community and the benefits this brings to the host. There has therefore been an evolutionary pressure to acquire mechanisms beyond mucus coated gut epithelium that would avoid breaching of the gut barrier in the first place. The result is adaptive immunity, which brings specificity, flexibility and long-term memory.
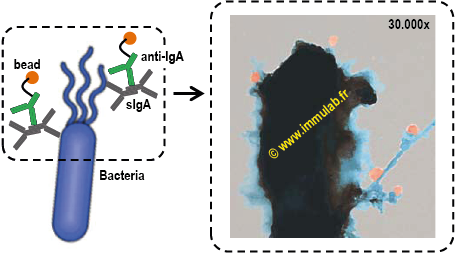 |
| Figure 1. Transmission electron microscopy of secretory IgA (sIgA) opsonized gut commensal. Purified gut commensals were stained with bead-conjugated anti-human sIgA antibody (50nm beads in orange). sIgA-binding of commensals was visualized with transmission electron microscopy. sIgA binds surface antigens, including fimbriae (adhesion), pili (reproduction) and flagella (motion). Image courtesy of Dr .Martin Larsen. |
Ironically adaptive immunity is depending on the transfer and presentation of commensal antigens into gut associated lymphoid tissues (GALT) lining the gut epithelium. To control transfer of antigens the gut epithelium contains specialized sites (Peyer’s patches) for antigen uptake. Follicular dendritic cells located in the subepithelial dome of Peyer’s patches capture microbial antigens and migrate to the perifollicular area to promote differentiation of naive CD4+ T cells into non-proinflammatory T cells.4 These cells induce IgA class switch and production through a PD-1 mediated pathway, which includes activating B cells through cognate antigen-driven T–B cell interaction. After emerging from the Peyer’s patch germinal centers, high-affinity IgA class-switched B cells enter the circulation and migrate to the lamina propria of the intestine, where they differentiate into long-lived IgA-secreting plasma cells. Commensal bacteria and GALT derived T cells co-exist as a tightly auto-regulated community. GALT derived Th17 cells regulates the expression of polymeric Ig receptor (pIgR) and thus IgA secretion.5 Importantly, absence of bacteria in germ free mice results in reduced gut IL-17 and secretory IgA (sIgA) levels.6 Conversely, potential pathobionts in mice, such as clostridium species and segmented filamentous bacteria induce Tregs and IL-17 production in the gut, respectively.7-9 GALT derived IgA+ B cells and Th17 cells can target distant organs, mediating immunopathologies, such as experimental autoimmune encephalomyelitis (EAE).6, 10
Effect of commensal bacteria in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis (MS)
A large set of evidence support an extensive impact of GALT driven immune imbalance caused by the commensal microbiome in EAE, the generally accepted experimental mouse model for human MS. Modification of the gut commensal microbiota has been demonstrated to alter the clinical outcome of EAE in mice.11 Oral treatment of mice with antibiotics reduced EAE severity by diminishing pro-inflammatory responses and enhancing FoxP3+ Tregs that accumulated in mesenteric and cervical lymph nodes. Protection was associated with cytokine patterns favouring Th2-type polarization over proinflammatory Th1/Th17 polarization.12, 13
The effect of orally-administered probiotics in the control of murine EAE has been studied using different Lactobacillus and Bifidobacterium strains. Prophylactic treatment abrogated EAE clinical symptoms and therapeutic treatment reduced the severity of established EAE.14 Conversely, other commensals contribute to disease severity.15
Finally, certain non-pathogenic commensal bacteria naturally present in the gut can induce spontaneous EAE in a relapsing-remitting mouse model. Indeed, germ free (GF) mice had life-long protection against EAE, whereas colonization with specific pathogen free commensals at birth or even in adult GF mice was enough to induce Th17 responses and EAE.6
In summary, an understanding of adaptive immunity directed against commensal gut bacteria is largely lacking. Moreover, numerous recent scientific advances in the field of autoimmunity underline the urgent need of investigating the impact of the commensal microbiota on human autoimmune disease onset and evolution. Studies of commensal-host mutualism demonstrate a very tight regulation of the innate/adaptive immune balance to sufficiently control homeostasis of the commensal microbiota. Moreover, the regulatory and pro-inflammatory T cell balance in GALT tightly regulates antibody-mediated tolerance to gut microbiota. Altogether, this suggests that alterations of adaptive immunity observed in MS (see below) could result from dysbiosis. It is so-far unclear whether immune surveillance is uniform for all members of the microbiota or is determined independently for each bacterial species. I therefore hypothesize that immune-interactions with the gut microbiota is key to the understanding of MS immunopathology.
References
1.Chow, J. & Mazmanian, S.K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 7, 265-276 (2010).
2.Hooper, L.V., Littman, D.R. & Macpherson, A.J. Interactions between the microbiota and the immune system. Science336, 1268-1273 (2012).
3.Molloy, M.J. et al. Intraluminal Containment of Commensal Outgrowth in the Gut during Infection-Induced Dysbiosis. Cell Host Microbe14, 318-328 (2013).
4.Kawamoto, S. et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science336, 485-489 (2012).
5.Cao, A.T., Yao, S., Gong, B., Elson, C.O. & Cong, Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol189, 4666-4673 (2012).
6.Berer, K. et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature (2011).
7.Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science331, 337-341 (2010).
8.Ivanov, II et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell139, 485-498 (2009).
9.Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity31, 677-689 (2009).
10.Lee, Y.K., Menezes, J.S., Umesaki, Y. & Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A108 Suppl 1, 4615-4622 (2011).
11.Ochoa-Reparaz, J. et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol183, 6041-6050 (2009).
12.Matsushita, T., Horikawa, M., Iwata, Y. & Tedder, T.F. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol185, 2240-2252 (2010).
13.Wilson, M.S. et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol40, 1682-1696 (2010).
14.Lavasani, S. et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One5, e9009 (2010).
15.Nichols, F.C. et al. Unique lipids from a common human bacterium represent a new class of Toll-like receptor 2 ligands capable of enhancing autoimmunity. Am J Pathol175, 2430-2438 (2009).